DNA Interface :
Write your name with your DNA
Course Project: MAS.S61 How To Grow (Almost) Anything, MIT, 2024
Synthetic Biology, Speculative Design, Prototyping Futures
THIS PAGE WILL BE UPDATED SOON
- PLEASE CHECK OUT THE NOTION PAGE UNTIL THEN (CLICK)
Archive of the class projects including sessions and labs (Link)
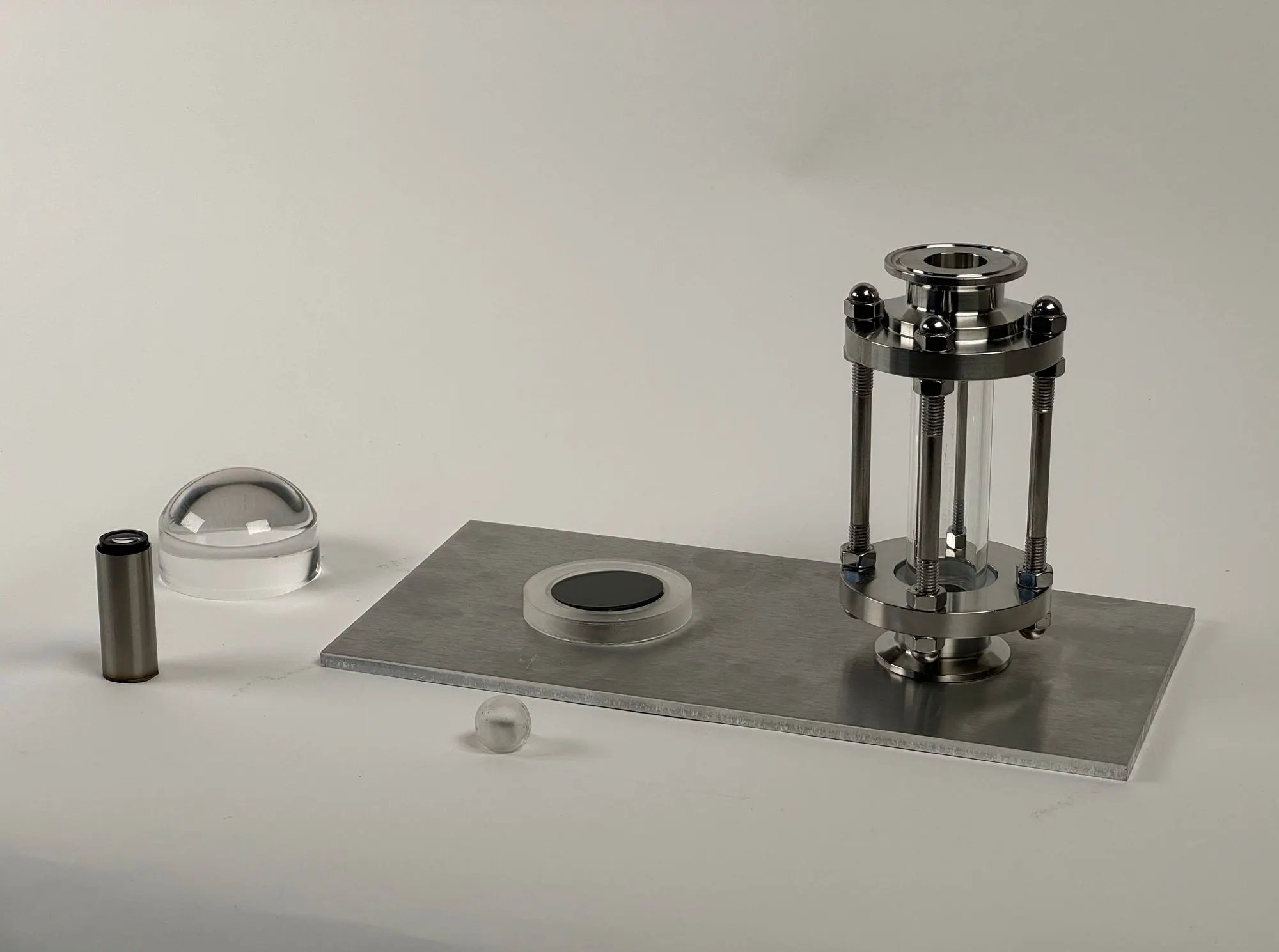


DNA Interface - Three phases of DNA Interfaces
- Write your name with your DNA
- Hold your Emotion - What’s the physical form of emotion?
- Physical Twin of your body - What if we have a ‘ball’ made of actual DNA, protein, and everything we have interacting in sync with our actual body?
Inspiration
 Vesper, Sci-fi Film
Vesper, Sci-fi Film1. Write Your Name with Your DNA
The use of DVespa also inspire futuristic applications of DNA interfaces.
Exploring the concept of a 'DNA interface,' I envision a future where DNA can be used to store personal information, such as names or family pictures, and even synthesize hormones to physicalize human experiences or emotions. This innovative approach aligns with key research in the field, such as studies on fast and modular DNA computing by Chatterjee et al., and the semantics of DNA-based computation by Lakin and Phillips. Dalchau and colleagues' work on the synthesis and tuning of chemical reaction networks using DNA further illustrates the potential of this technology, providing a strong foundation for integrating DNA interfaces into interactive wearable devices.
Reference papers:
- Gourab Chatterjee, Neil Dalchau, Richard A. Muscat, Andrew Phillips, Georg Seelig. "A spatially localized architecture for fast and modular DNA computing." Nature Nanotechnology, 2017.
- Matthew R. Lakin, Andrew Phillips. "A strand graph semantics for DNA-based computation." Theoretical Computer Science, 2016.
- Neil Dalchau, Niall Murphy, Rasmus Petersen, Boyan Yordanov. "Synthesizing and tuning chemical reaction networks with specified behaviours." International Conference on DNA Computing and Molecular Programming, 2015.
2. Hold Your Emotion - What’s the Physical Form of Emotion?
What is emotion, anyway? What’s the physical form of emotion?
In my project "Headspace," I explored the idea of physicalizing emotions through interactive and immersive installations. This concept can be extended using DNA-based interfaces to regulate and manifest emotional states. Researchers have developed DNA circuits and biosensors capable of detecting and responding to biochemical markers, paving the way for advanced health monitoring and therapeutic applications. For instance, hybridization chain reactions (HCR) have been employed to create highly sensitive biosensors that can detect specific RNA sequences within living cells, enabling precise monitoring of physiological states and emotional biomarkers.
My speculative DNA interface envisions using DNA to not only store emotional data but also to synthesize hormones that correspond to specific emotions. This approach builds on research showing the potential for DNA-based systems to interact dynamically with our biology, allowing us to physically manifest and regulate emotions. This aligns with recent studies on bioorthogonal DNA circuits, which demonstrate high specificity and efficiency in tracking intracellular biomarkers, providing a foundation for creating intricate and responsive biological interfaces.

Reference papers
- Feiyu Wang et al. "Recent Advances in Functionalization Strategies for Biosensor Interfaces." Chemosensors, 2023.
- Yingying Chen et al. "Bioorthogonal regulation of DNA circuits for smart intracellular microRNA imaging." Chemical Science, 2021.
- Recent progress in the development of DNA-based biosensors integrated with hybridization chain reaction or catalytic hairpin assembly. Frontiers in Bioscience, 2022.
3. Physical Twin of Your Body
The potential for DNA-based interfaces extends beyond data storage to applications such as creating physical representations of biological states. Researchers have developed DNA circuits and biosensors capable of detecting and responding to biochemical markers, paving the way for advanced health monitoring and therapeutic applications. Hybridization chain reactions (HCR) have been employed to create highly sensitive biosensors that can detect specific RNA sequences within living cells, enabling precise monitoring of physiological states.
Building on this, my speculative DNA interface envisions a 'ball' made of actual DNA, proteins, and other biological components that interact in sync with the human body. Such an interface could serve as a physical twin, reflecting and responding to the body's biochemical and emotional states. This concept is supported by recent research on bioorthogonal DNA circuits, which demonstrate high specificity and efficiency in tracking intracellular biomarkers, providing a foundation for creating intricate and responsive biological interfaces. Additionally, advancements in localized hybridization chain reactions and catalytic hairpin assemblies have shown significant improvements in sensitivity and specificity, further enhancing the capabilities of DNA-based biosensors.

Reference papers:
- Feiyu Wang et al. "Recent Advances in Functionalization Strategies for Biosensor Interfaces." Chemosensors, 2023.
- Yingying Chen et al. "Bioorthogonal regulation of DNA circuits for smart intracellular microRNA imaging." Chemical Science, 2021.
- Recent progress in the development of DNA-based biosensors integrated with hybridization chain reaction or catalytic hairpin assembly. Frontiers in Bioscience, 2022.
- Feiyu Wang, Yiwen Xie, Weijie Zhu, Tianxiang Wei. "Recent Advances in Functionalization Strategies for Biosensor Interfaces." Chemosensors, 2023.
- Chen, Gong, Gao et al. "Bioorthogonal regulation of DNA circuits for smart intracellular microRNA imaging." Chemical Science, 2021.
Q. What’s the meaning of embedding information into the DNA from ourselves?
Lab Protocol for the first aim
- Write your name with your DNA
- Write my name with DNA, and read it by sequencing the DNA
A. Extract DNA from your body
Protocol : 2022 DNA Extraction exercise + 2024 CRISPR exercise(Not done yet)
Isolate DNA - Lab Kit
Work time: 1-1.5 hours.
Use a DNA extraction kit (Qiagen DNeasy® Blood & Tissue Kit)
Purification of total DNA from animal saliva using the DNeasy® Blood & Tissue Kit, User developed protocol: https://www.qiagen.com/gb/resources/download.aspx?id=22471a48-832e-488d-8be6-2b308133b88a&lang=en
Note: This procedure has been adapted by customers from the DNeasy animal blood and cell protocol and is for purificatn of DNA from fresh or frozen animal saliva using the DNeasy Blood & Tissue Kit. It has not been thoroughly tested and optimized by QIAGEN.
IMPORTANT: Please read the “Safety Information” and “Important Notes” sections in the DNeasy Blood & Tissue Handbook before beginning this procedure. For safety information on the additional chemicals mentioned in this protocol, please consult the appropriate material safety data sheets (MSDSs), available from the product supplier. DNeasy Blood & Tissue Kits are intended for research use. No claim or representation is intended to provide information for the diagnosis, prevention, or treatment of a disease.
Equipment and reagents to be supplied by user
- DNeasy Blood & Tissue Kit (cat. no. 69504 or 69506)
- Pipets and pipet tips
- Vortexer
- Microcentrifuge tubes (1.5 ml or 2 ml)
- Microcentrifuge with rotor for 1.5 ml and 2 ml tubes
- Centrifuge capable of attaining 1800 x g with 15 ml falcon tubes
- Thermomixer, shaking water bath, or rocking platform for heating at 56°C
- Ethanol (96–100%)*
- PBS, pH 7.2 (50 mM potassium phosphate, 150 mM NaCl)
Important points before starting
- If using the DNeasy Blood & Tissue Kit for the first time, read “Important Notes” in the DNeasy Blood & Tissue Handbook.
- All centrifugation steps are carried out at room temperature (15–25°C).
- Vortexing should be performed by pulse-vortexing for 5–10 s.
- PBS is required for use in steps 1 and 2. Buffer ATL is not required in this protocol.
Things to do before starting
- Buffer AL may form precipitates upon storage. If necessary, warm to 56°C until the precipitates have fully dissolved.
- Buffer AW1 and Buffer AW2 are supplied as concentrates. Before using for the first time, add the appropriate amount of ethanol (96–100%) as indicated on the bottle to obtain a working solution (note: this will already have been completed by the TA).
- Preheat a thermomixer, shaking water bath, or rocking platform to 56°C for use in step 4.
Collect your saliva sample at home before just before coming to the lab
- Avoid eating/drinking at least 30 minutes prior to saliva collection.
- Rub your tongue along your gums to ensure you slough off plenty of genetic material.
- Collect 1ml of saliva in your provided eppendorf tube. Clean the outside of the tube with isopropanol, place the tube in a plastic bag, and bring it with you to lab.
DNA Extraction Protocol
-
Spray the outside of your saliva tube with ethanol and wipe with a kimwipe. Carefully parafilm the sample lid to keep it closed while heating and place your sample in a 92°C heat block for 15 minutes to inactivate any possible SARS-2-CoV contamination. Change gloves. Allow to cool for 5 minutes.
-
While the sample is cooling, add 4 ml PBS to a 15 ml falcon tube. Once cooled, add 1 ml of the heat-inactivated saliva, mix well. Transfer 2 ml sample to two 2 ml eppendorf tubes and centrifuge at 1800 x g for 5 min.
Ensure centrifuge is properly balanced! Ask a TA if you have questions.
-
Carefully decant the supernatant. Evenly divide the remaining sample among the two tubes. Centrifuge at 1800 x g for 5 min.
-
Carefully decant the supernatant. Resuspend each of the two pellets in 90 μl PBS, mix well. Transfer both samples to a single 1.5 ml eppendorf tube.
-
Add 25 μl proteinase K solution and 200 μl Buffer AL. Mix thoroughly by vortexing, and incubate at 56°C for 10 min.
It is essential that the sample and Buffer AL are mixed immediately and thoroughly by vortexing or pipetting to yield a homogeneous solution.
-
Add 200 μl ethanol (96–100%) to the sample and mix thoroughly by vortexing. It is important that the sample and the ethanol are mixed thoroughly to yield a homogeneous solution.
-
Pipet the mixture from step 5 (including any precipitate) into the DNeasy Mini spin column placed in a 2 ml collection tube (provided). Centrifuge at ≥6000 x g (8000 rpm) for 1 min. Discard flow-through and collection tube.
-
Place the DNeasy Mini spin column in a new 2 ml collection tube (provided), add 500 μl Buffer AW1, and centrifugefor 1 min at ≥6000 x g (8000 rpm). Discard flow- through and collection tube.
-
Place the DNeasy Mini spin column in a new 2 ml collection tube (provided), add 500 μl Buffer AW2, and centrifuge for 3 min at 20,000 x g (14,000 rpm) to dry the DNeasy membrane. Discard flow-through and collection tube. It is important to dry the membrane of the DNeasy Mini spin column, since residual
may interfere with subsequent reactions. This centrifugation step ensures that no
ethanol will be carried over during the following elution. Following the centrifugation step, remove the DNeasy Mini spin column carefully so that column does not come into contact with the flow-through, since this will result in ethanol. If carryover of ethanol occurs, empty the collection tube, then reuse it in centrifugation for 1 min at 20,000 x g (14,000 rpm).
-
Place the DNeasy Mini spin column in a clean 1.5 ml eppendorf tube (not provided), and pipet 100 μl water directly onto the DNeasy membrane. Incubate at room temperature for 1 min, and then centrifuge for 1 min at ≥6000 x g (8000 rpm) to elute. This is your DNA sample!
**** CAUTION: DO NOT ADD BLEACH TO FLOW-THROUGH!!! Flow-through contains Buffer AL or Buffer AW1 and is therefore not compatible with bleach. Discard flow-through in a falcon tube in the hazardous waste box. See DNeasy Blood & Tissue Handbook for safety information.****
Now that I’m writing this, I thought I should have taken a selfie of me trying to spit into a tiny centrifuge tube.






Prep - PCR






DNA extraction

DNA Sample from my saliva
B. Encode your information into DNA

It was too long to put into below 60 base pairs limits.
Some chat with GPT for shortening down the length of the sequence.
Eventually I orderd from IDT



FInal code for encoding information in DNA / and read the information into ASCII
dna1.py
ascii.py
WIP - using nltk to shorten the sequence
dna_nltk.py
Acknowledgement!! Huge thanks to TA Steven Ness!

C. Design a Plasmid with Benchling - put your name in it!
GGTGCCTCCGTGTCTGTTGTTGGT → Where should I put this?
Option 1)
cut puc19 / follow the protocol -> gibson assembly -> circular plasmid with your name
Option 2)
CRISPR

CRISPR Prime Editing Plasmids
*Source: Originally CRISPR Lab protocol, https://www.nature.com/articles/s41467-021-25541-3#Sec22*go to the editing point
- cut / add a single base
- single-based mutation
- or cut. insert a big chunk
Reference research material
**https://www.addgene.org/browse/article/28216983/**
-
mpubMed
-
Full text link
-
Supplementatary materials
41467_2021_25541_MOESM1_ESM.pdf

[notes from Eyal]
gtcctaggtataatactagtCTTGTCACTACTCTGACCTAgttttaga gctagaaatagc
modular way to do whatever you want
Uppercase part -> address : this part is GFP. ——> CTTGTCACTACT
first lower case letters —> copy and search. perfect fit
you can change the capital letters -> they’re in the different plasmid
CTTGTCACTACTCTGACCTA-> GFP
change the address to be something human
but -> what’s interesting is everything is same. you don’t touch lowercase, uppercase -> edit
First things -> complementary to what you’re editing.
CTTGTCACTACTCTGACCTA (PEgRNA_GFP)
-
TCACTACTCTGACCTATTAAGGT GTTCAA (PEgRNA_GFP_TA Ain_R)
bind to the target
TCACTACTCTGACCTATTGTAGAATCAGCCCACGAAACCGAGCGGGCGAGGGTGTTCAA
99 / 90 max
limit to order from idt
90
- maybe try three different primers
FWRD -> we have somewhere
for the reverse -> information.
edit GFP - turn off
TCACTACTCTGACATATCTTTCAAAG

[more notes] Difference of,,,
- Puc19 - empty container : to hold the piece of DNA. / grow it forever.
- pPegRNA - has something. promoter -> guide RNA scaffold —> put into bacteria it will produce RNA
plasmids ->
puc19 is same without gRNAscaffold and
CRISPR -> turn into RNA
specific shape -> folds / captures protein
DNA. editing. changing genome.
CRISPR -> find the target. RNA. inserts an edit
theoratically -> put into other people’s DNA
inside the nucli (let’s say your cell) -> it will DNA

shut off the computer
CRISPR -> in vivo system. it’s still working. you don’t need to open up the cell.
So, Primers I need
PEgRNA_GFP_F
gtcctaggtataatactagtCTTGTCACTACTCTGACCTAgttttagagctagaaatagc
- For PCR amplification of desired PEgRNA functional cassettes. Sequences in lower case are overhangs for Gibson assembly.
pVRb_PEgRNA_backbone_F
TTTTTTTGAAGCTTGGGCCC
- For PCR amplification of plasmid
pVRb_PEgRNA_backbone_R
ACTAGTATTATACCTAGGACTGAGC
- backbone for Gibson assembly of PEgRNA.
Primer_rev_FIrst_Name(based on PEgRNA_GFP_33i n_R)
gggcccaagcttcaaaaaaaTCACTACTCTGACCTATGGTGCCTCCGTGTCTGTTGTTGGTGGTGTTCAAgcaccgactcggtgccactt
- based on PEgRNA_GFP_33i n_R, edited for embedding my name into the DNA
- order both PAGE / non-PAGE options
Primer_rev_initial(based on PEgRNA_GFP_33i n_R)
HTG_Final_Primer_rev_FirstName_PAGE
Order from IDT
Acknowledgement!! Huge thanks to Eyal!


[Future Lab works]
- Do the other half of the lab - CRISPR
- Transformation and Plating (Miniprep, Gibson Assembly, Transform)
- Culture
- Sequence -
Acknowledgement!! Huge thanks to Evan! Helped me with initial ideation and figuring out what I need to order [notes from Evan below]
- Genomic DNA extraction kit (for your saliva).
- DNA from Twist to encode the information you want.
- IMPORTANT: the DNA information needs to have recognition sites for all of the restriction enzymes you’ll use (these are put at the beginning and end of the DNA sequence)
- Restriction enzymes (BsaI and PaqCI are common ones that are used in 2-enzyme systems). The following are required in addition to the enzymes, usually sold in a kit:
- PaqCI activator
- T4 ligase
- T4 ligase buffer
and of course more thoughts on,,,

Highly Specific Detection of Oxytocin in Saliva
Muhit Rana,1 Nimet Yildirim,1 Nancy E. Ward,2 Stephanie P. Vega,2 Michael J. Heffernan,2 and Avni A. Argun1,*
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10003004/
(Thanks again to Steven!)

Small form factor DNA Sequencer
https://www.ox.ac.uk/news/2015-10-15-mini-dna-sequencer’s-data-belies-its-size